Is Stainless Steel OK with Sulfuric Acid?
Stainless steel is a versatile material known for its corrosion resistance and durability. However, when it comes to sulfuric acid, a common industrial chemical, the question arises: is stainless steel okay with sulfuric acid? Let’s delve into the details to understand the compatibility between these two substances.
Understanding Sulfuric Acid
Sulfuric acid, also known as oil of vitriol, is a highly corrosive and strong acid. It is widely used in various industries, including chemical manufacturing, metal processing, and food processing. Sulfuric acid is available in different concentrations, ranging from 10% to 98%, with 98% being the most concentrated form.
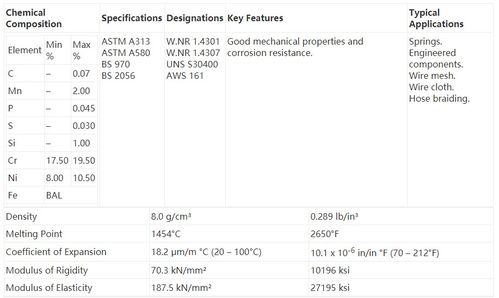
Stainless Steel Composition

Stainless steel is an alloy of iron, carbon, and other elements, primarily chromium. The presence of chromium in stainless steel provides it with excellent corrosion resistance properties. The minimum chromium content in stainless steel is typically 10.5%, but higher percentages can enhance its resistance to corrosion.
Compatibility Between Stainless Steel and Sulfuric Acid

When it comes to the compatibility of stainless steel with sulfuric acid, it is essential to consider the concentration of the acid and the type of stainless steel. Here are some key points to keep in mind:
-
Low Concentration Sulfuric Acid: Stainless steel is generally okay with low concentration sulfuric acid (up to 10%). In this case, the corrosion rate is relatively low, and stainless steel can be used without any significant issues.
-
High Concentration Sulfuric Acid: As the concentration of sulfuric acid increases, the corrosion rate also increases. At 98% concentration, stainless steel may experience significant corrosion, leading to pitting and localized corrosion. In such cases, alternative materials like Hastelloy or Inconel may be more suitable.
-
Stainless Steel Type: The type of stainless steel plays a crucial role in its compatibility with sulfuric acid. Austenitic stainless steels, such as 304 and 316, are generally more resistant to corrosion than ferritic or martensitic stainless steels. However, even these austenitic grades may experience corrosion at high concentrations of sulfuric acid.
Corrosion Mechanism
The corrosion mechanism of stainless steel in sulfuric acid involves the formation of a passive film on the surface of the material. This passive film acts as a barrier, preventing further corrosion. However, at high concentrations of sulfuric acid, the passive film may become unstable, leading to active corrosion.
Preventive Measures
When using stainless steel in the presence of sulfuric acid, it is essential to take certain preventive measures to minimize corrosion:
-
Use of Inert Gases: Inert gases like nitrogen or argon can be used to displace the sulfuric acid vapor, reducing the corrosion rate.
-
Controlled Temperature: Lowering the temperature can decrease the corrosion rate of stainless steel in sulfuric acid.
-
Regular Cleaning: Regular cleaning of the stainless steel surface can help remove any sulfuric acid deposits, reducing the risk of corrosion.
Conclusion
In conclusion, stainless steel can be used with sulfuric acid, but its compatibility depends on the concentration of the acid and the type of stainless steel. At low concentrations, stainless steel is generally okay, but at high concentrations, alternative materials may be more suitable. Taking preventive measures can help minimize corrosion and extend the lifespan of stainless steel in the presence of sulfuric acid.
| Stainless Steel Grade | Chromium Content (%) | Corrosion Resistance |
|---|---|---|
| 304 | 18-20 | Good |
| 316 | 16-18 | Excellent |
| 430 | 16-18 | Good |













